Effects Of Wrong Procedure In Electrolysis Experiment
Electrolysis experiment is the process of reacting acid and metals together in order to have the release the ions of the metals into the acid either for the purpose of creating a small voltage that can be detected by galvanometers or for the purpose of electroplating a particular metal.
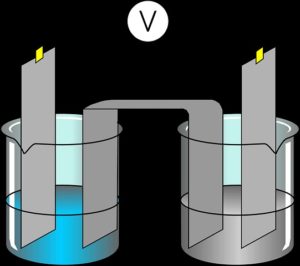
An electrolysis experiment that is meant to produce voltage will usually comprise two different metals with the one serving as the positive terminal and not reactive to the ions of the negative terminal.
An example of such is when a graphite rod (from a normal radio battery) acting as the positive terminal is inserted into a bow containing tetra-oxo-sulfate (iv)acid and zinc plates folded together acting as the negative terminal is also inserted in the same bowl at the opposite direction while the end of the wires of these two terminals is fixed to a galvanometer.
The result will be a release of hydrogen gas followed by the release of zinc ions into the bow solution while the galvanometer will be indicating the small voltage in the setup, which is usually from 0.1volts to 0.5 volts.
While the electrolysis experiment that is meant for electroplating would consist of two metals of different reactive strengths.
A solution of one of the metals to be used in the electroplating of the other will be set up in the same way the above electrolysis for voltage is set up, by having the bow filled with the solution of metal that would be electroplating other metals.
Example chromium is one of the metals normally used for electroplating, to electroplate an iron spoon with chromium, put chromium solution in a bow and insert a chromium rod into it as a negative terminal, and put the iron spoon into the solution at the opposite side as the positive terminal, then connect the terminals to a primary cell.
The result will be a rapid release of chromium ions into the chromium solution by the chromium rod while the iron spoon acting as the positive terminal will receive the ions and have its body covered by the ions. The longer it stays the thicker the coating of chromium on it.
In the course of performing these experiments, there are safety precautions one must put into consideration, and failure to observe them can cause the following;
Dry coughing
I conducted an electrolysis experiment in a laboratory and after the experiment, the list effects were what am going to discuss.
A cough that seemed as though something entered the walls of my throat with the little feeling of dryness in the throat, the situation lasted for about 20 minutes after the experiment.
Though I cannot categorically point out the cause of such a reaction I do suspect that I might have inhaled vaporized hydrogen gas coming out from the beaker I was using during the experiment.
Sneezing
I observed that the following day I started sneezing, the sneezing lasted for a whole day, that was when I decided to trace why such an experiment could lead to my sneezing but I could not exactly figure out why it happened.
The only thing that does come to my mind was that the laboratory is very small with only one small window and the only door it has was closed during the experiment in order to avoid distractions, maybe the vaporized gas might have filled the atmosphere in the laboratory such that I was inhaling it without knowing.
Irritation in the eyes
Another body reaction I observed was eye irritation just immediately after I finished the experiment but the reaction stopped later after I washed my face with water.
Though its duration was very short I concluded that I did not conduct the experiment properly, maybe I should have created a means for the gasses to escape easily out of the laboratory.
Skin reactions
I noticed itching on my skin though not a serious type but it lasted for some time even after washing my hands with soap.
With all these experiences and encounters I would recommend putting the following into consideration before conducting the electrolysis experiments;
Use rubber hand gloves
Wearing hand gloves will ensure that direct contact with the acids is completely avoided, which could help in eliminating skin reactions.
Cover the nose
Wearing a nose mask is another way of preventing the vaporized hydrogen gas from entering your nose if such a device is not available tying the nose with a neat cloth can perform the same function.
Wear eyeglass
Wearing eyeglasses of any kind will help to reduce the entrance of gasses into the eyes.
Keep distance from the bow
Keeping a good distance from the experiment area while observing the reactions and results is a better way of reducing any harmful effects the experiment would cause to you.
With all these, you may not have any problem each time the experiment is carried out irrespective of the duration of the experiment.
